
Lucas Pinto, MD, PhD
Assistant Professor
Department of Neuroscience
Feinberg School of Medicine
Northwestern University
lucas.pinto AT northwestern DOT edu
Lucas is an Assistant Professor of Neuroscience at Northwestern. He earned a medical degree from the Federal University of Minas Gerais, Brazil, in 2006. He then did a Master’s degree in physiology at the same university, working with Jerome Baron. Lucas completed his PhD in neuroscience from the University of California, Berkeley in 2014, working in Yang Dan’s laboratory. He moved to the Princeton Neuroscience Institute in 2015 for his postdoc in the laboratories of David Tank and Carlos Brody. Scroll down for an overview of Lucas’s CV, and graduate and postdoctoral research.
Curriculum Vitae
EDUCATION
12/2014 PhD in Neuroscience, University of California, Berkeley, CA, USA
Supervisor: Prof. Yang Dan
Dissertation: Extrasensory components of sensory discrimination
03/2009 MSc in Physiology, Federal Univ. of Minas Gerais, Belo Horizonte, Brazil
Supervisor: Prof. Jérôme Baron
Thesis: Neuronal responses properties related to motion processing in the owl visual wulst
12/2006 MD, Federal Univ. of Minas Gerais
POSITIONS & AFFILIATIONS
09/2022 Affiliated faculty, Department of Neurobiology, Weinberg College of Arts and Sciences, Northwestern University. Evanston, IL, USA.
01/2021 Assistant Professor, Department of Neuroscience, Feinberg School of Medicine, Northwestern University. Chicago, IL, USA.
08/2015 – 12/2020 Post-doctoral fellow, Princeton Neuroscience Institute, Princeton University, Princeton, NJ, USA. Labs of David Tank and Carlos Brody
01/2015 – 06/2015 Post-doctoral fellow, Helen Wills Neuroscience Institute, University of California, Berkeley, CA, USA. Lab of Yang Dan.
SELECTED AWARDS
2024 NSF CAREER Award.
2022 Scialog Molecular Basis of Cognition Fellow.
2022 Sloan Research Fellow.
2021 Allen Institute Next Generation Leader.
2019 K99/R00 BRAIN Initiative Advanced Postdoctoral Career Transition Award from the National Institute of Mental Health.
2017 F32 National Research Service Award from the National Institute of Neurological Disorders and Stroke.
2013 F31 Ruth L. Kirschstein National Research Service Award from the National Institute of Neurological Disorders and Stroke.
2009 Milton I. and Florence Mack Neurology Research Fellowship (UC Berkeley)
Read more about my graduate and postdoctoral work below

We are constantly making decisions. However, not all decisions are created equal. For example, when driving in traffic on a clear day, fast sensory processing of a red traffic light is enough to trigger the appropriate decision of stepping on the brakes. But on a stormy night the same decision may require prolonged staring through a water-splashed windshield. In other words, the driver needs to add an intermediate, time-extended computation to accumulate the noisy and ambiguous sensory evidence of the red light. In my postdoc I set out to answer the question of what are the differences in the large-scale cortical mechanisms supporting decisions that require different underlying computations.
In collaboration with other BRAIN CoGS postdocs, I first developed a novel virtual navigation-based task for mice to study decisions that require the gradual accumulation of sensory evidence, as well as similar tasks that do not require evidence accrual. I then combined the tasks with a suite of optical techniques to manipulate and record dorsal cortical activity on meso- to large spatial scales. My findings suggest that complex decisions engage widespread processes throughout the entire dorsal cortex, with different areas performing different computations. Simpler decisions, in turn, engage more spatially localized computations.
I am currently following up on these results using more temporally specific inactivations of different cortical areas, in isolation or combination. Combined with computational models, this will help us understand how different cortical areas act in concert to mediate evidence-accumulation-based decisions.
Our behavioral state fluctuates constantly. It does so both on long timescales throughout the sleep-wake cycle and on much faster, seconds-long timescales. These fluctuations profoundly impact how we perceive and act upon the world. Think, for example, of the difference between paying attention to and spacing out during a lecture. I am interested in understanding how brain states are controlled and the mechanisms by which they alter perception and decision-making. A large part of my PhD work focused on the impact of cholinergic projections from the basal forebrain on sensory discrimination and cortical activity patterns. Using a combination of behavior, multisite extracellular recordings, anatomical tracing and optogenetics, we showed that these projections can bidirectionally modulate visual perception and coding in the visual cortex on sub-second timescales, such that increases in cholinergic activity result in better behavioral performance and enhanced sensory coding. They do so by decorrelating cortical activity and increasing the reliability of sensory-evoked activity, improving the signal-to-noise ratio of V1 responses. We have also shown through calcium imaging of genetically identified cell types that the effects of manipulating cholinergic projections are compatible with how the activity of cell bodies in the basal forebrain is modulated by perceptual decision-making behavior.
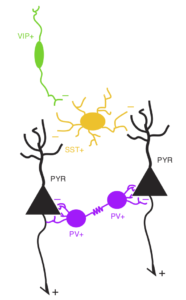
The prefrontal cortex (PFC) plays a crucial role in coordinating sensory, motor and cognitive processes for goal-directed behavior. The response properties of its neurons have been extensively studied, with findings generally pointing to functional heterogeneity and complex, mixed selectivity. How these properties are computed by the PFC microcircuit, however, is much less understood. To study this during my PhD, I used a combination of behavior, pharmacology and microendoscopic calcium imaging from genetically identified cell types in the mouse dorsomedial PFC. First, we showed that this region is required for performance of a sensory discrimination task. Moreover, we found that inhibitory interneurons of the same subtype were similar to each other, but different subtypes preferentially signaled different task-related events: somatostatin-positive neurons primarily signaled motor action, vasoactive intestinal peptide-positive neurons responded strongly to action outcomes, whereas parvalbumin-positive neurons were less selective, responding to sensory cues, motor action, and trial outcomes. Compared to each interneuron subtype, pyramidal neurons showed much greater functional heterogeneity, and their responses varied across cortical layers. Such cell-type and laminar differences in neuronal functional properties may be crucial for local computation within the PFC microcircuit, and provide important constraints for future computational models of PFC function.
Here’s a video of me talking about this work, produced by Inscopix.
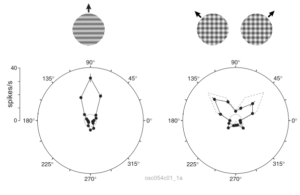
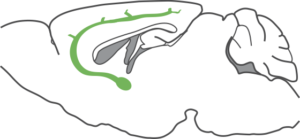
The visual wulst is a bird brain structure analogous to the mammalian visual cortex. It is particularly well developed in owls, reaching 25% of brain volume in some species. Besides a comparative neuroscience perspective, studying this structure is interesting because the wulst and the mammalian visual cortex do not share a common evolutionary origin. In other words, they are the fruit of convergent evolution — solutions found independently by the different species. Therefore, they shed light on the general principles and constraints underlying visual processing in neural circuits. My MSc work shows that owl visual wulst neurons act like localized spatiotemporal filters (see also this) that do not signal speed or global object motion, much like their mammalian V1 counterparts.
